Semaglutide's injectable formulation offers a convenient diabetes management option, requiring careful handling and specific storage (2°C to 8°C) away from sunlight and heat sources. Mismanagement, like incorrect cooling or exposure to light/moisture, can render it ineffective. Proper storage, tracking expiration dates, aseptic administration, and safe disposal are crucial for maintaining safety and efficacy. Following these guidelines ensures each injection delivers the intended therapeutic effect.
Learn how to effectively store and handle semaglutide injections with this comprehensive guide. Discover the intricacies of the semaglutide injectable form, explore storage considerations for optimal effectiveness, and understand ideal conditions for safe preservation. We’ll also cover handling techniques to minimize degradation, tracking inventory, common mistakes to avoid, and best practices for disposal. Additionally, we address frequently asked questions, offering insights tailored to your storage concerns.
Understanding Semaglutide Injectable Form: A Brief Overview
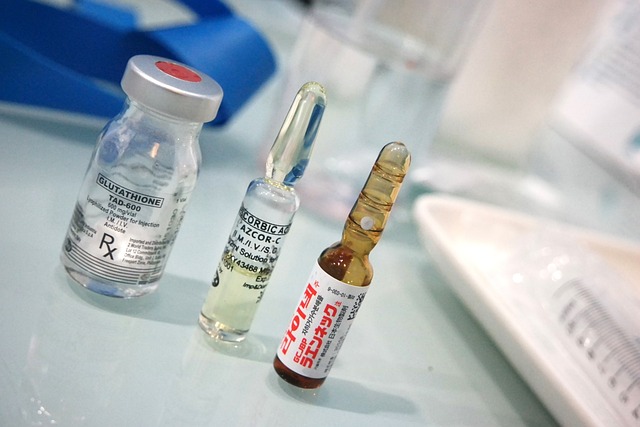
Semaglutide, a medication commonly used for diabetes management, is available in an injectable form that offers a convenient and effective treatment option. Understanding the unique properties of semaglutide’s injectable formulation is essential for proper storage and handling to ensure its efficacy and safety. This form typically comes as a solution or suspension, designed for subcutaneous injection, allowing for better glycemic control.
The injectable semaglutide is usually supplied in vials or pre-filled syringes, requiring careful handling to maintain its stability. Proper storage conditions, including temperature and exposure to light, play a crucial role in preserving the medication’s potency. Patients and healthcare providers should be aware of these requirements to ensure the medicine remains effective when administered, providing consistent benefits for diabetes management.
Storage Considerations for Optimal Effectiveness

To ensure optimal effectiveness and stability, semaglutide injections should be stored under specific conditions. This medication is typically provided in an injectable form, making proper storage crucial for maintaining its quality and potency. It’s recommended to keep semaglutide out of direct sunlight and heat, storing it instead in a cool, dry place. Refrigeration is usually the best option, but check with your pharmacist or healthcare provider for any specific instructions regarding temperature ranges.
Additionally, avoid freezing the medication as this can cause it to become ineffective. Always inspect the semaglutide injectable form before use; check for any visible signs of damage, discoloration, or separation of components, which could indicate its stability has been compromised. Following these storage considerations will help guarantee that each injection provides the intended therapeutic effect.
Ideal Conditions for Safely Storing Semaglutide

To ensure the safety and efficacy of semaglutide injections, it’s crucial to maintain ideal storage conditions. The semaglutide injectable form should be stored in a cool, dry place, away from direct sunlight and heat sources. A temperature range of 2°C to 8°C (35°F to 46°F) is recommended to preserve the medication’s potency. Proper storage protects the drug from degradation caused by exposure to light or excessive heat, which can alter its chemical structure and reduce its effectiveness.
Additionally, keep semaglutide out of reach of children and in a secure location to prevent accidental ingestion. Never store it in the bathroom, as fluctuating temperatures and moisture levels can compromise the medication’s integrity. Always follow the instructions provided by your healthcare provider or the drug manufacturer for proper handling and storage practices to ensure optimal results when administering semaglutide injections.
Handling Techniques to Minimize Degradation

Proper handling techniques are essential to maintain the potency and stability of semaglutide injections, ensuring their effectiveness for diabetes management. The semaglutide injectable form is sensitive to light, heat, and air, which can accelerate its degradation. To minimize these factors, always store the medication in its original packaging, keeping it away from direct sunlight and excessive heat sources. Refrigeration is recommended to prolong the drug’s shelf life; maintain a consistent temperature between 2°C and 8°C (35°F and 46°F) to prevent degradation.
When preparing an injection, handle the vials and syringes carefully to avoid exposure to light and air. Draw up the required dose slowly and steadily, minimizing air bubble formation in the syringe. Once drawn, ensure the needle is properly sealed to prevent oxidation and subsequent loss of efficacy. Discard any unused medication safely according to local guidelines to avoid contamination and maintain a consistent supply of effective semaglutide for your treatment regimen.
Tips for Tracking Expiry Dates and Inventory

Staying organized is key when managing semaglutide injections. Regularly check and track expiry dates is essential, especially as the semaglutide injectable form has a specific shelf life. Create a simple inventory system to monitor your supply; this can be done using a dedicated app or even a spreadsheet. Note down the quantity of vials or pens you have, their expiration dates, and when they were received. This way, you’ll never run out of your medication and can discard expired vials promptly.
Visual cues are helpful reminders. Keep a well-stocked medicine cabinet or dedicated refrigerator for your semaglutide supplies, clearly labeling each item with its expiry date. Regularly review your inventory, disposing of any outdated medications safely according to local guidelines.
Common Mistakes to Avoid During Storage and Administration

Storing and handling semaglutide injections incorrectly can lead to reduced efficacy or even spoilage. A common mistake is misstating the expiration date, as proper storage conditions must be maintained up until the end of the specified period. Another error is improper cooling; while semaglutide injectable form should be refrigerated, direct freezing should be avoided to prevent degradation. Users often make the mistake of exposing the medication to excessive light or moisture, which can also compromise its quality.
During administration, healthcare providers and patients frequently overlook the need for aseptic techniques, risking contamination and potential adverse reactions. Additionally, incorrect dosages are a significant concern; always follow prescribed guidelines and consult a healthcare professional if unsure about administration methods.
Best Practices for Disposal of Used Injectables

Proper disposal of used semaglutide injectables is crucial to maintain safety and environmental sustainability. After each use, it’s essential to dispose of the syringes and needles as hazardous waste, following local regulations. Many communities have specific guidelines for discarding these items, which may include securing them in rigid containers or bringing them to designated collection points.
To prevent accidental exposure and contamination, used semaglutide injectables should never be thrown away with regular trash. Additionally, ensure that the disposal site is far from water sources, as proper management of these waste materials helps protect ecosystems and prevents potential harm to wildlife.
Frequently Asked Questions: Addressing Storage Concerns
Many patients and healthcare providers have concerns about storing and handling semaglutide injections, especially since it’s available in an injectable form. Frequently asked questions address common storage worries. It’s important to keep semaglutide out of direct sunlight and extreme temperatures, as these conditions can affect its stability. Refrigeration is typically recommended, but remember that not all formulations require cold storage. Always check the medication’s labeling for specific instructions tailored to your particular brand and dosage.
To maintain peak potency, it’s crucial to follow guidelines on handling and administration. Avoid shaking the vial vigorously, as this might lead to particle settlement. Instead, gently invert the vial several times before each injection to ensure uniform suspension. Discard any visible particles of sediment, and never use if the solution appears cloudy or discolored. Promptly return unused portions to the refrigerator, ensuring they remain sealed to protect against environmental contaminants.
